ÉQUIPE REGPEP - DOMAINE DE COMPÉTENCE
Gut-microbiota - brain axis in regulation of motivated behavior (PI : Pr Serguei Fetissov)
A new concept (Figure 1) explaining molecular mechanisms of the communication in the gut microbiota - brain axis has postulated a role of bacterial mimetic proteins of neuropeptides (1). It was triggered by the key finding in patients with eating disorders of autoantibodies directed against α-MSH, an anorexigenic neuropeptide (2) and later complemented by discovery of the Escherichia coli protein ClpB responsible for the production of such antibodies (3). Moreover, a direct anorexigenic effect of ClpB was discovered (4), resulting in the development of the first probiotic to control appetite and body weight by the French start-up TargEDys (www.targedys.com). Our recent studies have extended this concept by discovery in Lactobacilli of a bacterial mimetic protein of oxytocin, a neuropeptide involved in regulation of stress and social behavior resulting in patent application and introduction by TargEDys of the new anti-stress probiotic preparation (5). Currently, we validate this protein as a putative molecular target in ASD in the frame of the H2020 European consortium GEMMA.
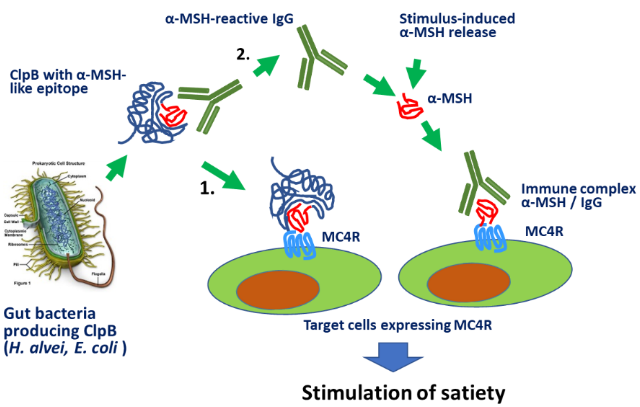
Moreover, a pathophysiological model of eating disorders has been proposed in which α-MSH-cross-reactive autoantibodies with altered capacity to bind α-MSH may play a key pathogenic role (6). Currently, we validate this model using the activity-based anorexia model in mice as well as clinical studies in the frame of the ERAnet consortium MIGBAN. Numerous experimental evidence has been obtained suggesting a role of neuropeptide-reactive IgG as a natural neuropeptide-carrier molecule which modulates the neuropeptide activation of its receptor (7). Our recent studies of oxytocin- and α-MSH- binding IgG further support such role of plasmatic IgG as a general phenomenon in peptidergic signaling.
[1] S. O. Fetissov, Neuropeptide-like signaling in the microbiota-gut-brain axis. Behavioral and Brain Sciences 42, e70 (2019).
[2] S. O. Fetissov et al., Autoantibodies against neuropeptides are associated with psychological traits in eating disorders. Proc Natl Acad Sci USA 102, 14865-14870 (2005)
[3] N. Tennoune et al., Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide [alpha]-MSH, at the origin of eating disorders. Transl Psychiatry 4, e458 (2014)
[4] R. Legrand et al., Commensal Hafnia alvei strain reduces food intake and fat mass in obese mice-a new potential probiotic for appetite and body weight management. Int J Obes 44, 1041-1051 (2020)
[5] M. Nicol et al., Lactobacillus salivarius and Lactobacillus gasseri supplementation reduces stress-induced sugar craving in mice. European Eating Disorders Review, (2023)
[6] S. O. Fetissov, T. Hökfelt, On the origin of eating disorders: altered signaling between gut microbiota, adaptive immunity and the brain melanocortin system regulating feeding behavior. Curr Opin Pharmacol 48, 82-91 (2019)
[7] S. O. Fetissov, M. El Mehdi, in Neuroendocrine-Immune System Interactions, J. P. Konsman, T. M. Reyes, Eds. (Springer International Publishing, Cham, 2023), pp. 187-204
FINANCEMENTS OBTENUS
- ERAnet NEURON project, “MiGBAN” – Microbiota Gut Brain Axis in Anorexia Nervosa. consortium of 5 EU partners coordinated Prof. Beate Herpertz-Dahlmann from Univ. of Aachen, Germany. https://www.ukaachen.de/kliniken-institute/migban/research-goal/
- H2020-BHC-03-2018 EC project consortium “GEMMA”- Genome, Environment, Microbiome & Metabolome in Autism. 17 EU and USA partners coordinated by Dr Alessio Fasano, from EBRIS, Salerno, Italy. https://www.gemma-project.eu/
- Transversal Microbiota Program of Inserm, including 20 partners from France, coordinated by Dr Nadine Cerf-Bensussan, Institut IMAGINE-INSERM 1163, Université Paris Descartes-Sorbonne Paris Cité, France
MEMBRES ACTUELS
- Serguei FETISSOV, PU
- Emilie LAHAYE, PhD student
- Benjamin THOMAS, Master student
TECHNIQUES ET MODÈLES EXPÉRIMENTAUX
- Bacterial proteomics
- Surface plasmon resonance
- Brain microdialysis, HPLC
- BTBR mice
PRÉCÉDENTS MEMBRES
- Rochellys Diaz Heijtz, Professor, group leader in Karolinska Institutet, Stockholm, Sweden
- Mouna El Mehdi, postdoc in Max Planck Institute for Metabolism Research, Cologne, Germany
- Marion Nicol, postdoc
- Ilaria Olivito, PhD student, University of Calabria, Cosenza, Italy
- Kuniko Takagi, postdoc
- Lisa Bremard, master student
- Jules Balance, master student

